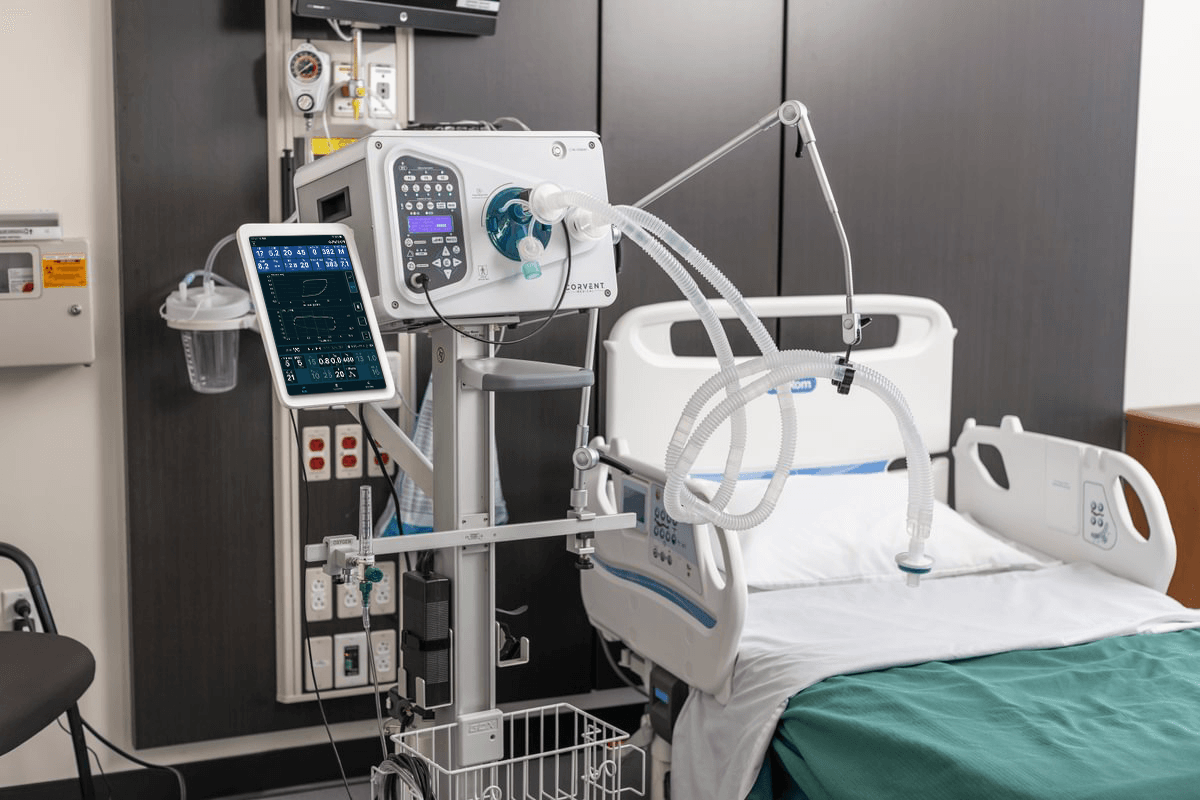
This year, CorVent Medical celebrates five years of innovation and impact on respiratory care. Since 2020, CorVent has remained committed to delivering dependable, easy-to-use technologies for routine, stockpile, and emergency ventilation, enabling providers to focus on what matters most: patient care.
A Timeline of Innovation
CorVent Medical was established in response to the COVID-19 pandemic, with a vision to revolutionize respiratory support by developing versatile, high-performance ventilators.
As we reflect on the past five years, we celebrate our journey and achievements.
- Became a top 5 finalist among 172 entries in the U.S. Department of Defense Hack-a-Vent Challenge, securing FDA Emergency Use Authorization (EUA) for the RESPOND-19 Ventilator.
- Relocated and expanded our U.S. headquarters and manufacturing to Fargo, North Dakota.
- Built a team of industry leaders and professionals committed to excellence and innovation.
- Achieved FDA 510(k) clearance and launched the RESPOND® Ventilator, a simple, safe, and smart solution for healthcare systems, providers, and patients.
Looking Ahead
As we continue to grow, our mission remains the same: to design and develop simple, safe, and smart respiratory solutions that improve patient outcomes.
“We are excited for the next phase of our journey, with new innovations, expanded partnerships, and a deepened commitment to supporting healthcare professionals. We will continue to grow, develop, and manufacture in the US innovative respiratory products and solutions with impactful results for both healthcare providers and their patients,” concluded Richard S. Walsh, president and CEO, CorVent Medical.