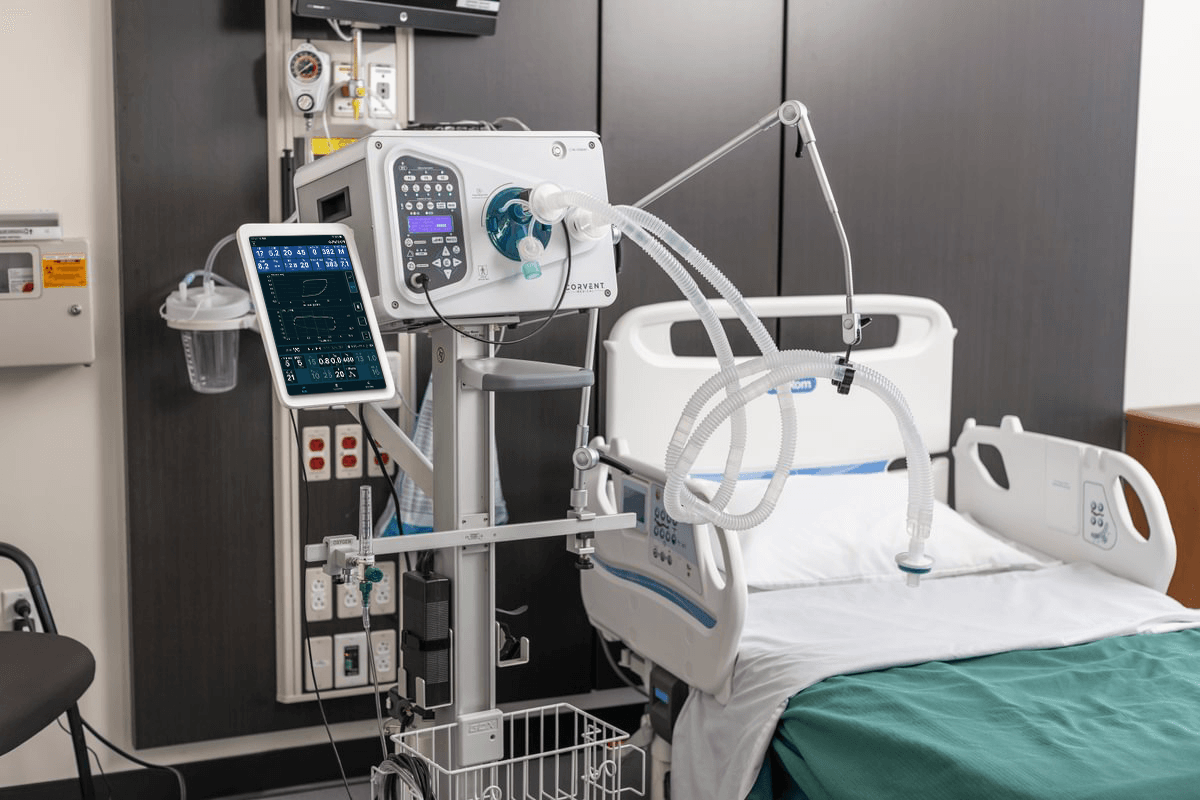
On April 6, 2022, CorVent Medical announced the successful certification of its facilities in the U.S. under ISO 13485:2016 certification by GMED, a French Notified Body, for the quality management system for design, development, manufacture, and distribution of adult ventilator systems for continuous use in hospital. This issuance follows an extensive auditing process that evaluated CorVent’s maintenance of the EC certification (CE marking) to EU Product, Health, and Safety standards, as well as proper implementation and maintenance of a quality management system.
Compliance with ISO 13485 demonstrates to customers and distributors that CorVent Medical conforms to strict quality systems that are often basic requirements to distribute medical devices internationally. Along with our CE Mark under MDD regulations, CorVent Medical has the necessary quality and regulatory approvals to continue distributing its premier product line, the RESPOND Ventilator, in the EU.
RESPOND Ventilators are available and ready to ship to countries within the EU – call 833-770-VENT (8368) or fill out a Request Form to learn more.